What is Phoslock?
Phoslock was developed in Australia by the Commonwealth Scientific and Industrial Research Organisation (CSIRO), to remove phosphates from water. The active element in Phoslock is lanthanum (a rare-earth element) which has a strong affinity to bind with phosphate. This forms an insoluble and biologically inert compound, Rhabdophane. Phoslock is comprised of 95% bentonite and 5% lanthanum.
It is manufactured through a controlled ion-exchange process whereby cations within the bentonite are exchanged with lanthanum cations. The result is that lanthanum held within the bentonite structure retains its ability to bind phosphate, but does not readily dissociate, i.e. will not form free ions in water.
Phoslock is manufactured as a dry granule which makes it easy to transport and store.
Jump to:
How is Phoslock applied to a water body
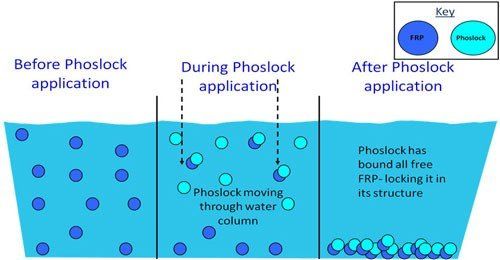
How does Phoslock work?
Phoslock works by utilising the ability of lanthanum to react with phosphate. Removal of phosphate by lanthanum is highly efficient and has a molar ratio of 1:1 which means that one ion of lanthanum will bind with one ion of phosphate. This binding forms the mineral Rhabdophane (an insoluble and biologically inert compound) which strips phosphate from the water.